Company Overview
Antisense Therapeutics Ltd (ASX:ANP) is an Australian publicly listed biotechnology company, developing and commercializing antisense pharmaceuticals for large unmet markets in rare diseases. The products are in-licensed from Ionis Pharmaceuticals Inc. (NASDAQ: IONS), an established leader in antisense drug development.
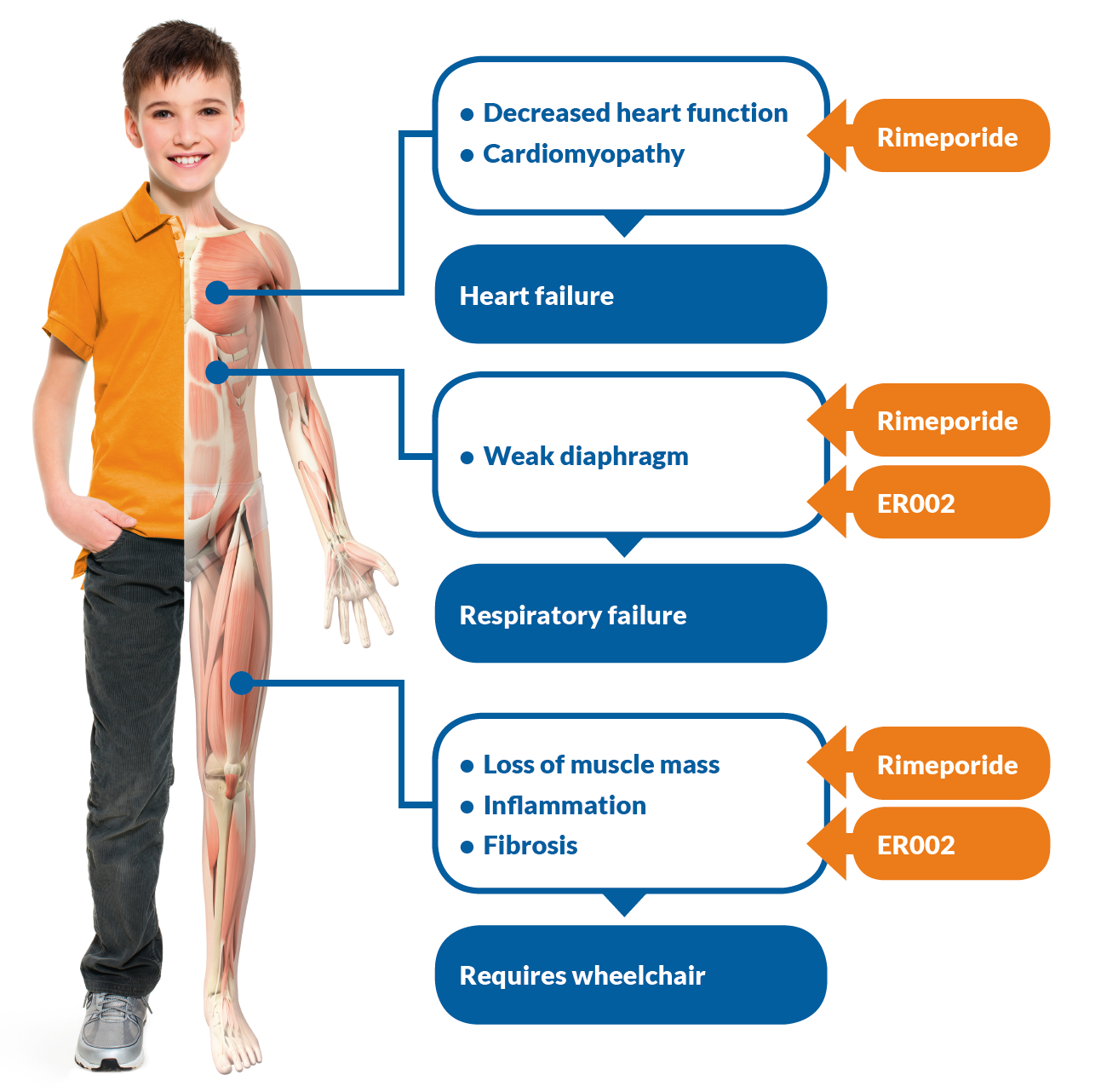
The Company's flagship program is ATL1102 which primarily services the market of Duchene's Muscular Distrophy Disease (DMD) patients. Duchenne muscular dystrophy (DMD) is a rare and fatal genetic muscle wasting disease that affects boys. At current, ATL1102 is the only therapy in development for DMD targeting CD49d to slow disease progression by reducing inflammation and resultant muscle damage with potential application across a broad range of inflammatory diseases.